Clinical Research: Hot Topics and Best Practices
July 27, 2022
We polled the audience at a recent Gardner Law continuing legal education event and learned that approximately 75% of attendees work at medical technology and pharmaceutical companies that are currently involved in clinical research initiatives. This jibes with our recent assignment load, which has been heavy on clinical research matters. Therefore, we thought it a good time to share some best practices on clinical research activities.
Click here to view a recording of the program on YouTube that covers:
- Clinical trial agreement review 101
- Recent OIG Advisory Opinion (22-05) on study subject cost-sharing
- Compensation dos and don'ts
- Recent Ocugen FDA Notice of Noncompliance for FDAAA research reporting concern
No time to watch the video? What follows is a brief summary of the video presentation.
Clinical trial agreement review 101
Although clinical research contracting can be arcane, the same issues come up again and again. Issues include general contracting considerations (parties, compensation, term, description of services, and the like), but also include more complex topics relating to FDA, anti-fraud and privacy matters, as well as malpractice and product liability.
Although complex, there is no reason to be overwhelmed when it comes to negotiating clinical research agreements. Once a person gets the handle on the topic, it is not that difficult.
Basic Terms
A written agreement is needed. What goes into a typical clinical trial agreement? A lot. But, here are some of the main topics.
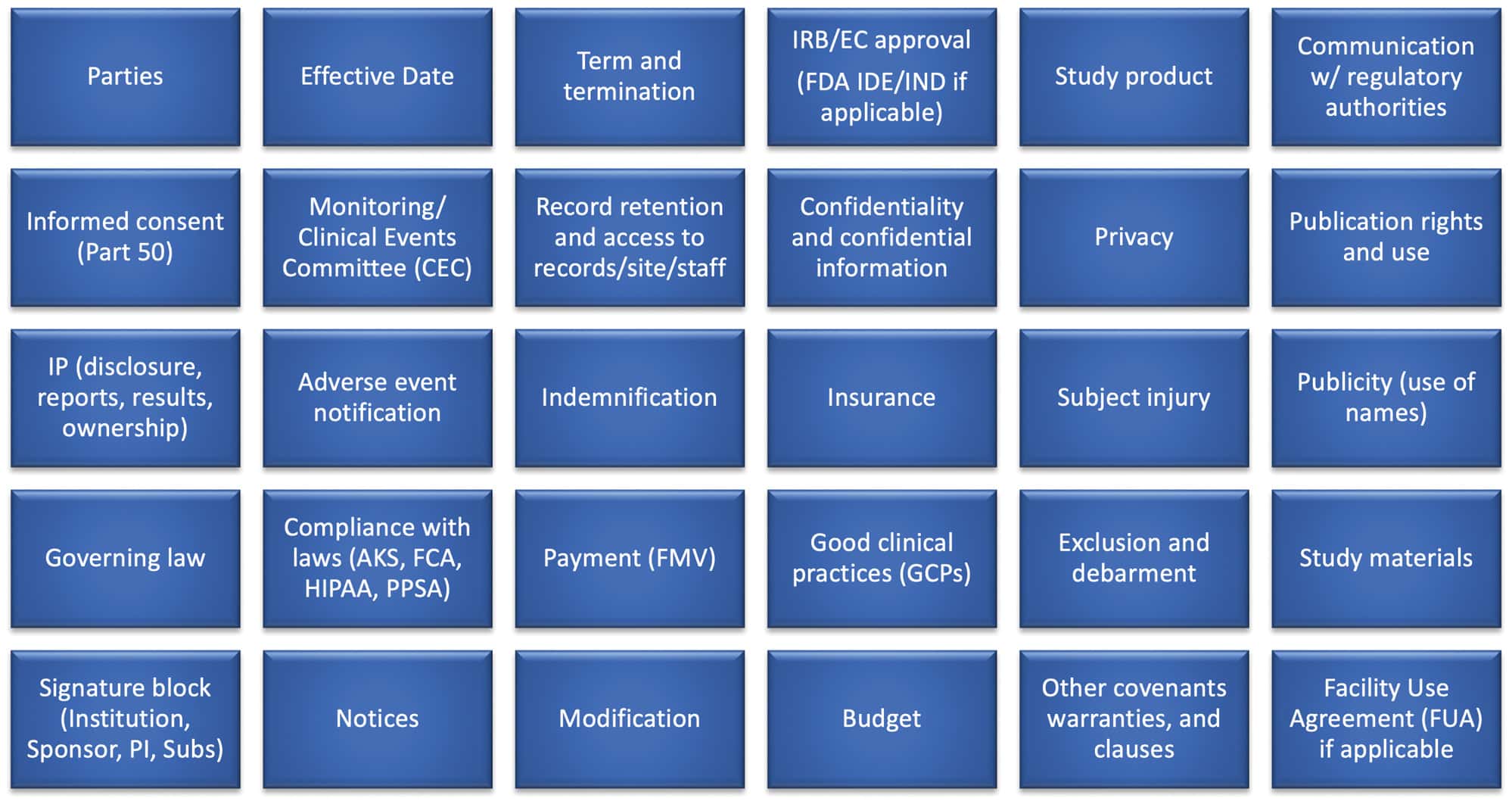
We could write a treatise on each of these subjects but we will spare our readers from such an exercise. Instead, here are a few salient points on each topic.
- Parties-these seem obvious, but name ALL of the parties to the agreement
- Effective date-be clear on when the contract goes into effect (e.g., date of execution)
- Term and termination-call out how long the agreement will last and how the parties can get out of it
- IRB (investigational review board), EC (ethics committee), and/or U.S. Food and Drug Administration (FDA) approval-call out the sponsor and who is responsible for such approval(s)
- Study product-outline what will be done with leftover product and consider any cost(s)
- Communicating with regulators-call out what happens in the event of regulator outreach (including, e.g., in the case of an FDA BIMO inspection)
- Informed consent-the informed consent form (ICF) must marry up with the agreement
- Monitoring-call out the party(s) responsible for monitoring and think about a CEC
- Records-outline how they will be kept, duration, and ownership/access rights
- Confidential information-define and place controls on it
- Privacy-consider local, state, federal and international requirements of this evolving area of law
- Publication rights-outline the rules for publication and rights for review
- IP-have your intellectual property attorney involved; carefully review federal cooperative research and development agreements (CRADA)
- Adverse event reporting-ensure responsibilities for monitoring, data analysis, risk assessment, and timely reporting, if necessary, are assigned
- Indemnification-watch out for edits to this contentious clause; it is best to gain bilateral indemnification so that one party is not responsible for the other's negligence
- Insurance-there are standards that should be in every contract
- Subject injury-determine how much liability the sponsor is willing to take on
- Publicity-outline how the company will use site and investigator names
- Governing law-pull in the corporate lawyer to determine if there is a preference
- Compliance-an overlooked subject on older agreements; make sure there is language in the contract that ties back to anti-kickback, false claims, and transparency laws
- Payment-outline terms and make sure payments meet the U.S. Federal Anti-Kickback Statute (AKS) Personal Services and Management Contracts and Outcomes Based Payment Arrangements (Personal Services) Safe Harbor
- GCPs-include a term that the parties agree to follow Good Clinical Practices
- Exclusion/debarment-do not contract with excluded or debarred parties
- Study materials-outline what these are and how they will be treated
- Signature block-this one seems obvious but consider how sub-principal investigators, for example, are covered by the research arrangement
- Notices-include where notices are to be sent, e.g., for termination, reporting, etc.
- Modification-include a process for changes
- Budget-consider how sites will be paid and what payments will be based on (e.g., Medicare rates); look out for excessive "overhead" fees, patient bounties, enrollment bonuses, and the like
- Other-consider what other basic terms may be needed, e.g., force majeure, counterparts, assignment, among others
- FUA-consider whether a site is going to request a Facility Use Agreement or "FUA"
These are just some of the basics. Each research arrangement is unique and therefore may require additional legal considerations. Talk to your attorney for more information. Involve them early on in the drafting process.
Controversial Terms
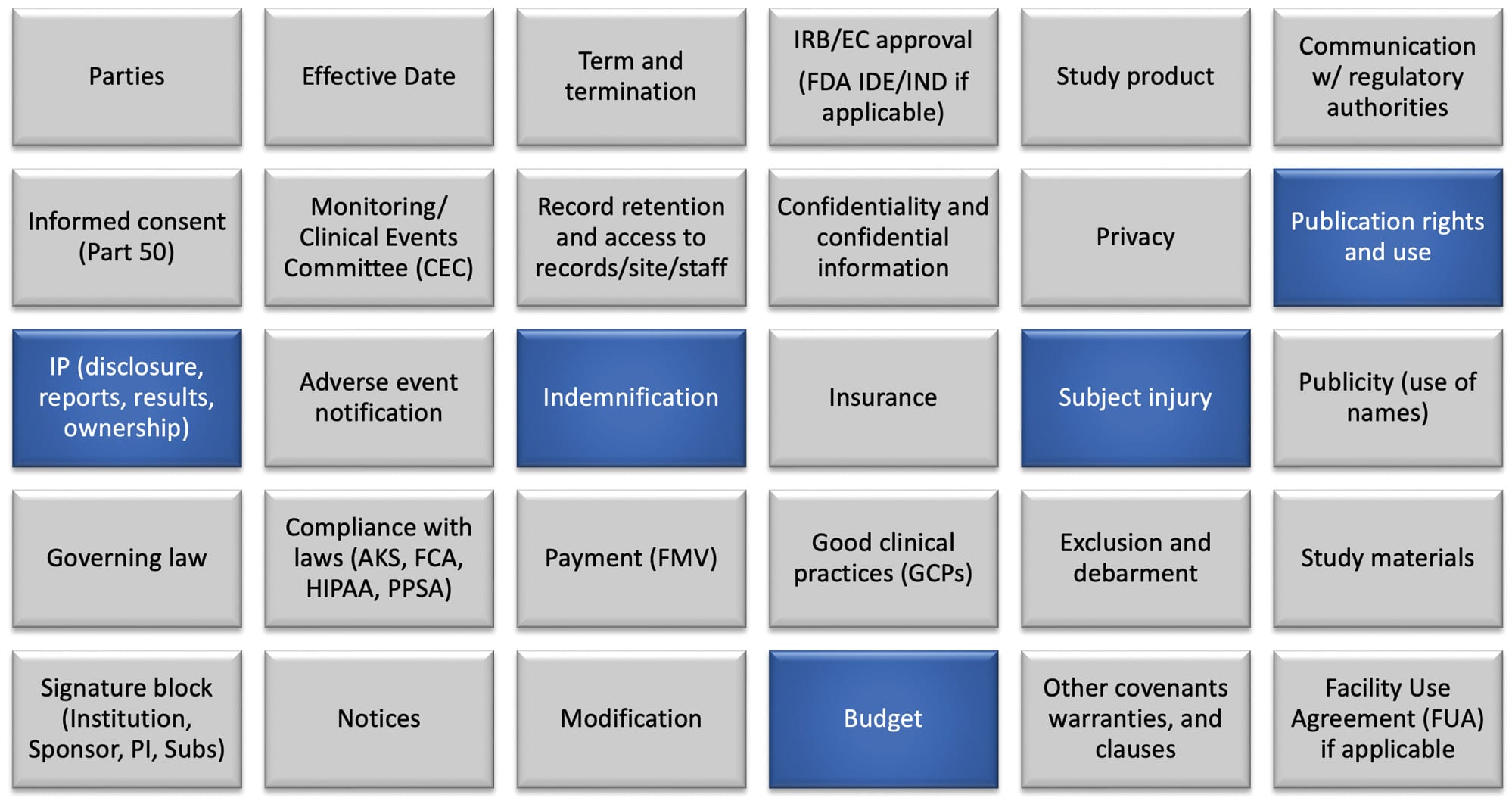
Some of the most contentious terms are highlighted in blue below. These terms deserve extra scrutiny in light of the impact they can have on a research arrangement. For example, failure to structure the publication rights section properly may limit how a sponsor can publish data or review a manuscript prior to publication. Changes made to terms related to intellectual property are common. Changes may impact the ability of a manufacturer to protect its intellectual property. Indemnification clauses are commonly edited. Try to get the "other side" to agree to a mutually equitable clause that does not go overboard. One of the most important and controversial sections in a clinical trial agreement is subject injury. Be careful to make sure your insurance carrier knows what you are agreeing to. The last thing a sponsor wants to do is take on more liability than it should. We have seen poorly written clauses abused. Budgets are also commonly an area of compromise. Have a basis for payments and be willing to walk away if you have to.
Talk through these contentious terms live with the other side. Getting on the phone and hammering out redlines can save time and be effective. Do not underestimate the "human touch" of negotiating in real time and getting to "yes" with the other research parties. Be patient with attorneys on the other end as greenhorns often are given the task of negotiating agreements with manufacturers. Take a conciliatory tone and play an educator role when necessary. Remember that everyone wants the same thing-to advance research objectives.
Click here to view the presentation on YouTube which discusses other controversial terms.
![cost sharing AdobeStock_484917181 [Converted]-01 cost sharing AdobeStock_484917181 [Converted]-01](/uploads/news/cost-sharing-AdobeStock_484917181-Converted-01.png)
Recent OIG Advisory Opinion (22-05) on Study Subject Cost-Sharing
The government recently considered the following question:
Are clinical trial patient care cost subsidies, which could induce Medicare beneficiaries to participate in a research study, and provide prospective payments to providers, unlawful and therefore subject to prosecution under anti-fraud laws (e.g., AKS, Civil Monetary Penalties (CMP) Law)?
In this case, the U.S. Department of Health and Human Services Office of Inspector General (OIG) took the position that even though the proposed arrangement did not meet any statutory exception or safe harbor, it condoned the arrangement because, among other reasons:
- The subsidies outlined in the proposed arrangement are a reasonable means of promoting socioeconomically diverse study subject enrollment and retention;
- The company would offer the same type of subsidies to subjects with Medicare and commercial insurance coverage;
- Payment would be made to the site/institution and not the beneficiary;
- The proposed arrangement poses a low risk of over-utilization or inappropriate billing to Federal health care programs because:
- The company would not advertise the availability of the cost-sharing subsidies (only outlined in the informed consent documents);
- Beneficiaries must satisfy the enrollment criteria and provide informed consent to be eligible to participate and thus be eligible for the subsidies;
- Investigators must comply with the Study Protocol, subject to IRB oversight and monitoring; and
- Study enrollment would be capped at 260 subjects; and
- The Proposed Arrangement is not a problematic seeding arrangement because the device in question is for a "one-time" treatment.
Click here to view the presentation on YouTube which discusses more details about the advisory opinion.
Note that OIG's conclusion in Advisory Opinion 22-05 exclusively applies to the requestor and is limited to the specific facts supplied. Therefore, while the opinion is informative to industry broadly, it is important for you to consult your attorney regarding your specific circumstances.
Any sponsor considering a similar arrangement should familiarize itself with the facts of Advisory Opinion 22-05 and solicit feedback from an attorney familiar with the AKS, U.S. Federal False Claims Act, U.S. Federal CMP Law, the U.S. Federal Food, Drug and Cosmetic Act, and the U.S. Federal Physician Payments Sunshine Act (Open Payments).
![budget AdobeStock_369407405 [Converted]-01 budget AdobeStock_369407405 [Converted]-01](/uploads/news/budget-AdobeStock_369407405-Converted-01.png)
Compensation dos and don'ts
Here are some helpful dos and don'ts for compensating researchers.
FMV (fair market value) assessment
- Follow the Personal Services Harbor to the AKS
- Research payments must be paid at FMV and cannot be a reward for purchases or referrals
- Review all payments in a budget to make sure they are legitimate
- Commercial staff stay out
- Consider what the basis is to set payments? (e.g., Medicare rates, purchased data)
- What about "overhead" payments? How much is too much?
Carefully draft your budget
- Payments should be itemized
- Make payments to the institution and not the investigators or staff
- Consider triggers for payment-if milestones are used then make sure they are achieved before paying
Frequently Asked Questions
Question: It is okay to pay for legitimate recruitment efforts?
Answer: Generally, yes. Pay fair market hourly rates negotiated at arm's-length and meet the Personal Services Safe Harbor.
Question: Is it okay to pay customer clinical trial staff a cash or in-kind bonuses (a.k.a., a bounty) in exchange for enrolling patients into a clinical trial?
Answer: This once common practice is frowned upon. Consider:
- The American Medical Association (AMA) asserts that "offering or accepting payment for referring patients to research studies (finder's fees) is unethical."
- AMA's prohibition of finder's fees in clinical research extends beyond its own membership, as many other entities require physicians to follow AMA's code of ethics in its entirety
- State laws (e.g., Ohio)
- IRB/EC rules
- See also: Offering Incentives, OEI-01-97-00195: Recruiting Human Subjects: Pressures in Industry-Sponsored Clinical Research; Recruitment Incentives, OEI-01-97-00196: Recruiting Human Subjects: Sample Guidelines for Practice; and HHS Guidance: Financial Conflict of Interest
Question: Is it okay to have a verbal agreement for payment of clinical research?
Answer: No. Draft an agreement that memorializes the terms of the research arrangement and meets applicable safe harbors. Consult with your legal counsel for more information and review of the contract.
Click here to view the presentation on YouTube which discusses more best practices.

Ocugen FDA Notice of Noncompliance
Under Section 801 of the Food and Drug Administration Amendments Act of 2007 (FDAAA) a responsible party for an applicable clinical trial is required to submit to the ClinicalTrials.gov data bank certain results information for the clinical trial. Such results information generally must be submitted no later than one year after the primary completion date of the applicable clinical trial. That is unless the responsible party has submitted a timely certification of delay, a request for an extension for good cause, or a request for a waiver of the requirements for submission of results information.
Ocugen, a small biotech company located in Pennsylvania, received a notice of noncompliance from the FDA Center for Drug Evaluation and Research (CDER) on April 15, 2022. More specifically, Ocugen received a letter from the Agency outlining "[n]oncompliance with the Requirements for Submission of Clinical Trial Results Information" for a phase 3 trial. Ocugen needed to post by May 15 and faces a civil money penalty of $10,000 "for each day of the violation" the submission is late. It is unclear whether the company has resolved the Agency's concerns. This enforcement action is a reminder to remain diligent with FDA reporting requirements.
Click here to view the presentation on YouTube which discusses more details about the Ocugen FDA Notice of Noncompliance.
Have questions? Contact us.
Information provided on this website is not legal advice. Communications sent to or from this site do not establish an attorney-client relationship. © 2022 Gardner Law. All Rights Reserved.