MMSEA Section 111 Reporting Obligations: Enforcement Is Here
September 30, 2025Why Sponsors Need to Act Now
Under Section 111 of the Medicare, Medicaid, and SCHIP Extension Act (MMSEA), sponsors that agree to cover research-related injuries may be classified as Responsible Reporting Entities (RREs). When a clinical trial participant is a Medicare beneficiary and the sponsor pays (or has a contractual obligation to pay) for medical expenses, that payment must be reported to the Centers for Medicare & Medicaid Services (CMS).
Failure to comply carries escalating risks, including Civil Money Penalties (CMPs) of up to $1,000 per day per claim for non-reporting or late reporting, along with the potential for regulatory scrutiny, contract disputes, and reputational harm.
What Must Be Reported for NGHPs:
- Payments (or a contractual obligation to pay) made by sponsors of clinical trials for complications or injuries arising out of a clinical trial must be reported where the injured party is a Medicare beneficiary.
- Information about payments (or obligations to pay) for medical care (“Medicals”) that are claimed or released, or a settlement/judgment/award (or other payment) that has the effect of releasing Medicals.
- In cases of Ongoing Responsibility for Medicals (ORM) (claims where the NGHP, such as a sponsor, continues to have responsibility for future Medicals), certain claims must be reported even if they are not yet resolved as of the reporting thresholds.
How / When Sponsors Must Report:
- Reporting must be done electronically via CMS’s Benefits Coordination & Recovery Center (BCRC).
- RREs may submit via full electronic file exchange or via manual Direct Data Entry using the CMS online COB Secure Website (COBSW), particularly if they have low claim volume.
- The claim information must be submitted for claims whose Total Payment Obligation to the Claimant (TPOC) date is on or after certain threshold dates (e.g. liability insurance claims with settlement etc. from Oct 1, 2011 onward) or earlier when ORM applies.
- RREs are required to register with CMS, test their file submissions, then begin submitting “production” files once approved.
Enforcement is Here:
- Final Rule (October 2023): CMS published regulations in the Federal Register outlining how and when penalties will be assessed against RREs.
- Effective Dates: The rule took effect in December 2023; CMS will begin assessing penalties in October 2025 for Group Health Plans (GHPs) and in January 2026 for Non-Group Health Plans (NGHPs), such as trial sponsors.
- Audit Framework: CMS announced it will conduct random audits each quarter (250 records sampled) to identify late or missing reports.
- Penalty Structure: CMPs range from $250 to $1,000 per day, per record, with maximum liability exceeding $365,000 per record depending on the reporting delay.
Although CMPs will only be issued prospectively, sponsors that fail to meet the timeliness requirements or that wait until 2026 to prepare may still be at risk.
“Section 111 compliance is not a box to check at the last minute—it requires systems, training, and contract clarity now. Early action safeguards your organization against costly penalties and ensures the integrity of your clinical trial operations.”
Brynn Stanley, Associate Attorney, Gardner Law

How Do You Know If You Are Required to Report?
Many sponsors are not aware they have triggered RRE status. If your CTA includes language agreeing to pay for medical treatment of research injuries, and the participant is (or becomes) a Medicare beneficiary and is injured as part of the study, you are likely required to report that information to CMS.
Here is what you need to watch for:
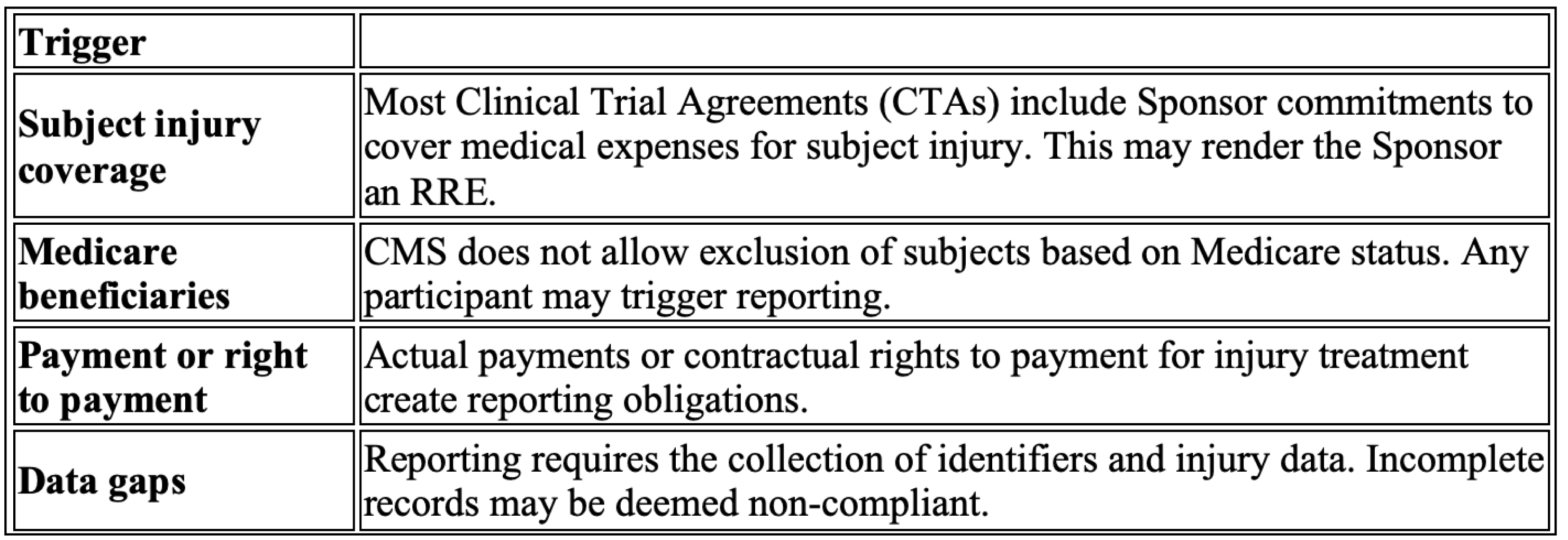
Risk Profile for Sponsors:
- Financial Exposure: Daily penalties can quickly accumulate across multiple trial participants or studies.
- Contractual Risk: CTAs may shift responsibility for reporting; unclear terms leave sponsors exposed.
- Operational Burden: Failure to implement reporting workflows now increases the risk of errors under CMS audits.
- Reputational Harm: Sites, IRBs, and regulators expect sponsors to meet statutory requirements, and lapses can undermine trust.
What Should Sponsors Do Now?
1. Conduct a compliance assessment
- Audit current CTAs for subject injury provisions.
- Map which trials or sites that may involve Medicare beneficiaries.
2. Establish reporting infrastructure
- Ensure agreements in place allow for Section 111 compliance, with the collection of Medicare IDs and injury details, as appropriate.
- Implement processes for timely CMS submissions.
3. Update contracts
- Clarify responsibility for Section 111 reporting in CTAs.
- Consider indemnities or insurance to allocate risk.
4. Train staff
- Train legal, clinical, regulatory, and finance teams to understand the reporting triggers.
5. Engage outside support
- Specialized counsel, such as Gardner Law, can help manage reporting obligations and prepare for CMS audits.
Conclusion
CMP enforcement of MMSEA Section 111 is no longer theoretical. With CMS’s penalty framework finalized and enforcement scheduled to begin in October, 2025 for GHPs and January, 2026 for NGHPs, sponsors must act now to mitigate risk. Reviewing subject injury provisions, confirming RRE status, and standing up compliant reporting systems are essential steps.
The cost of delay is high, but proactive compliance will protect both your organization and your clinical trial operations from financial and reputational risk.
If you have any questions about MMSEA Section 111 compliance, or if you would like assistance in MMSEA Section 111 reporting, contact Gardner Law.